View before and after photos with DUPIXENT IN ADULTS
to see results

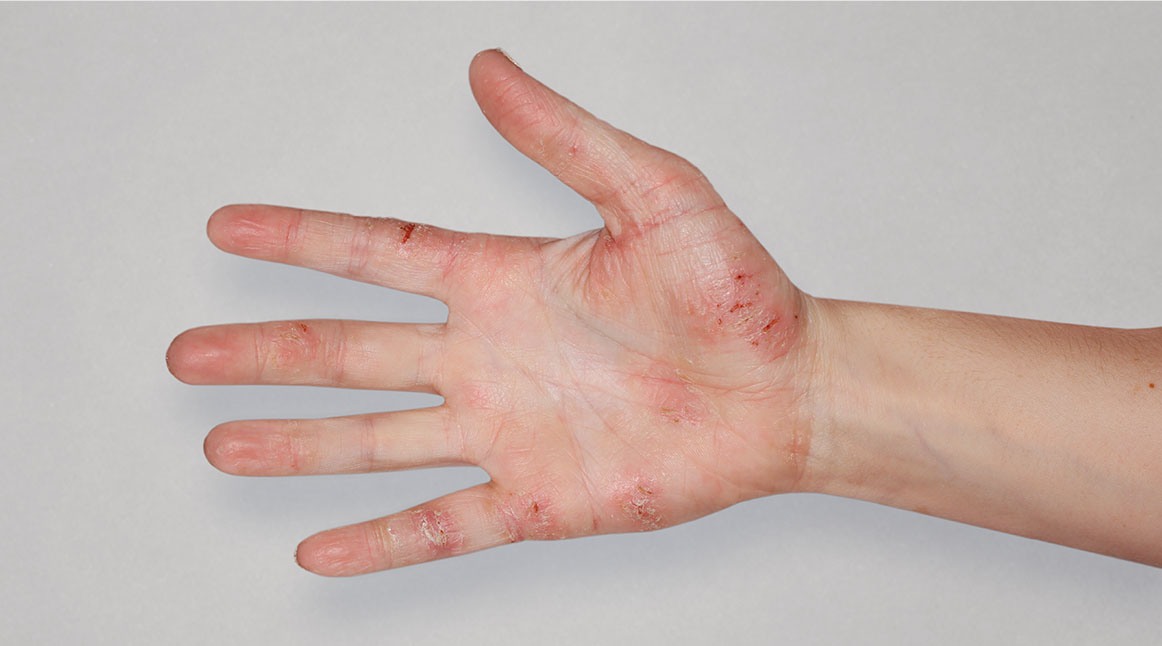
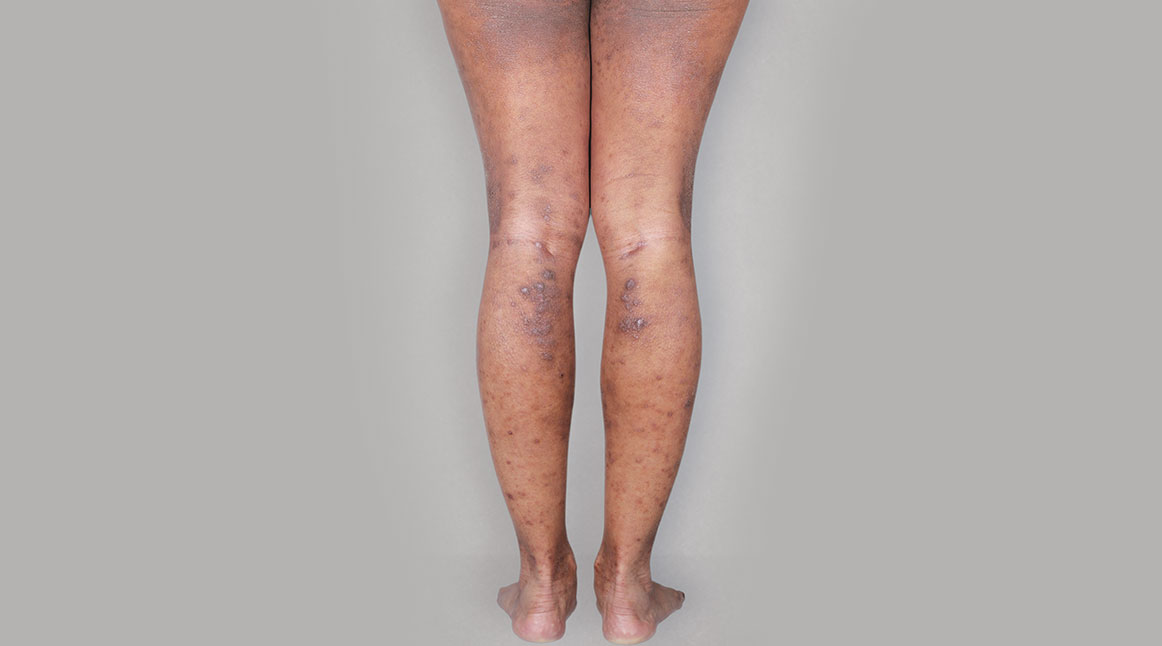
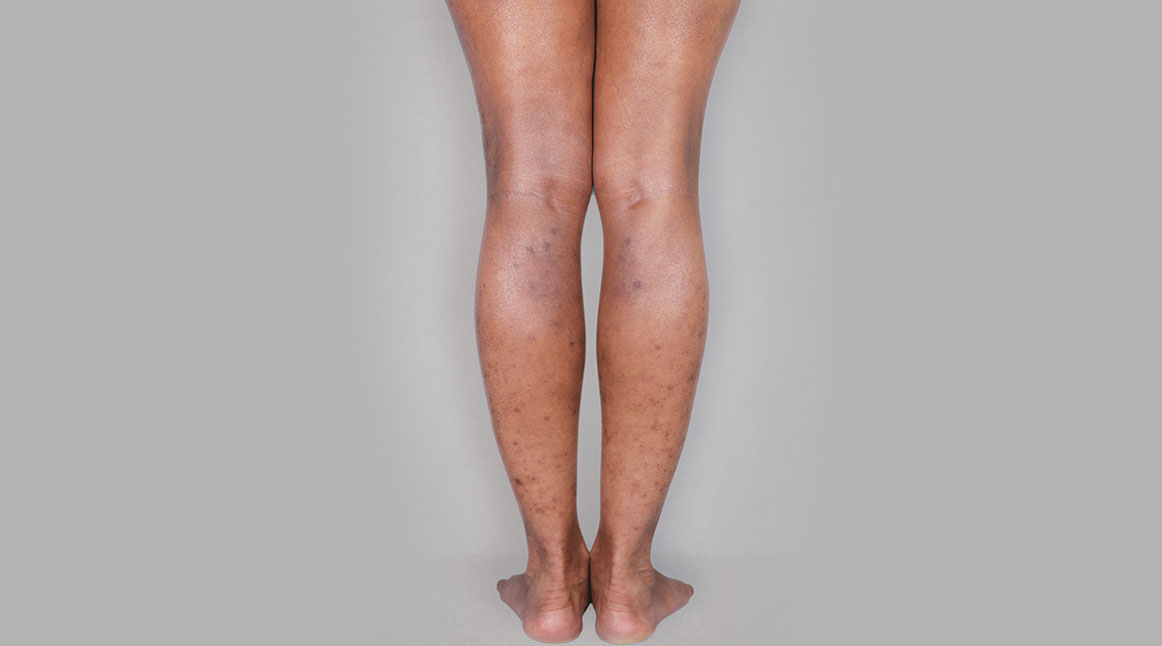
BeforeTreatment
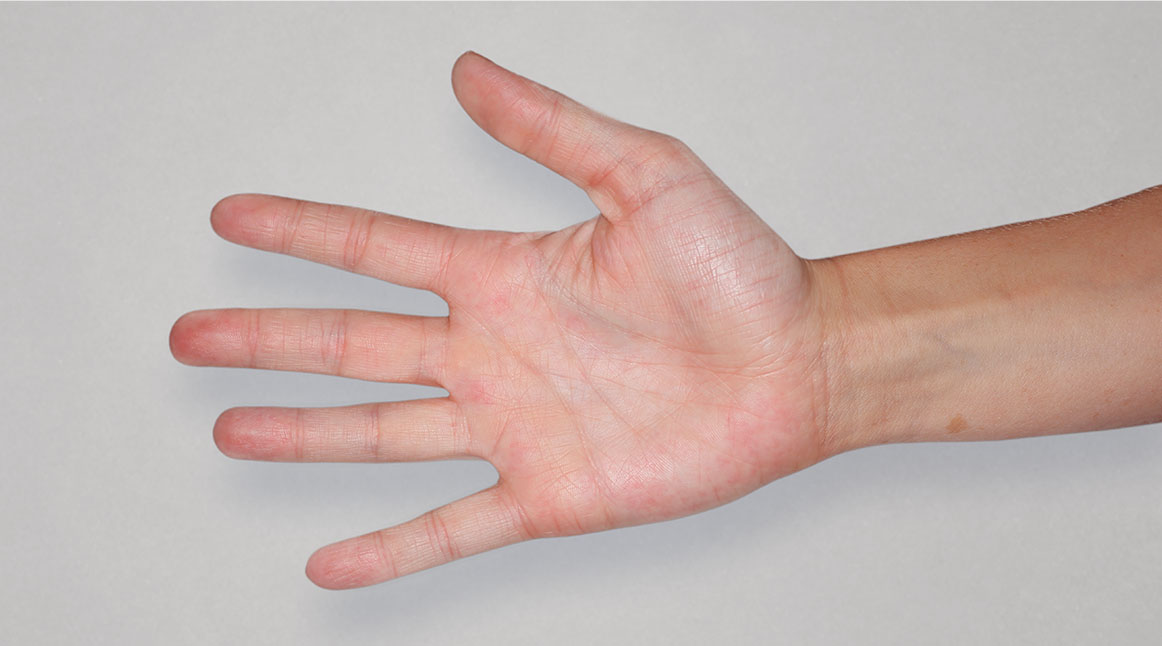
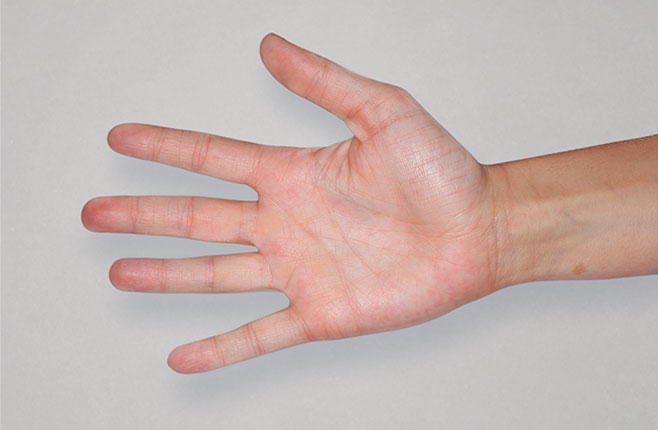
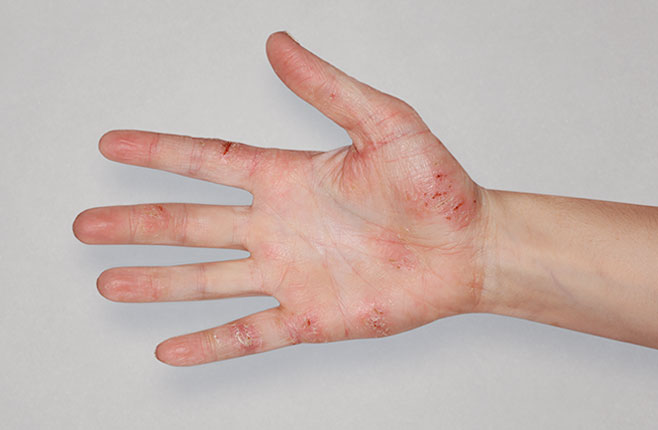
After Treatment
with dupixent
at 16 weeks
to see results

Before
Treatment
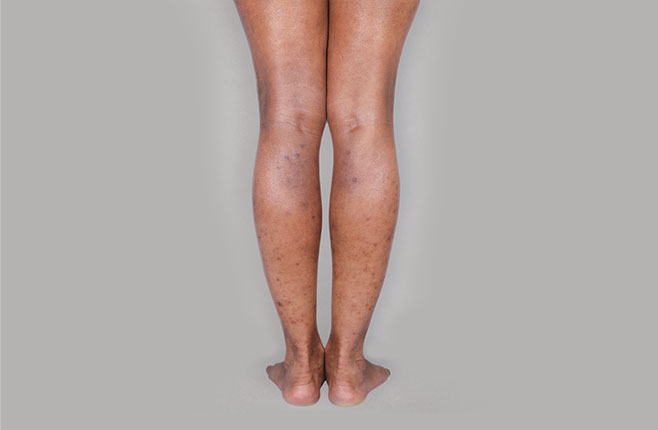
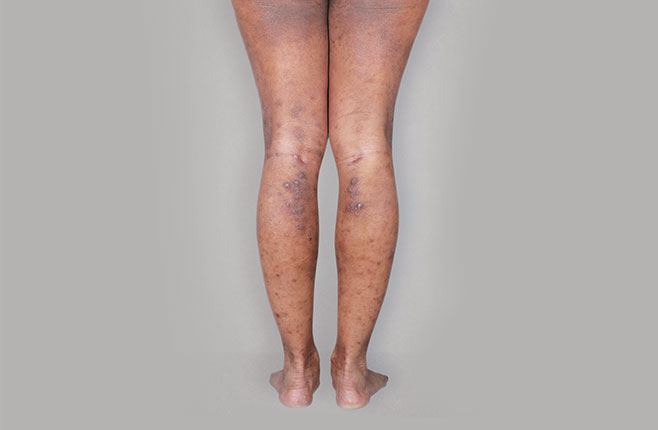
After Treatment
with dupixent
at 16 weeks
Actual real-world DUPIXENT patients shown; not clinical trial patients. Additional factors (including possible use of other treatments) may have impacted results. Individual results may vary.
Stay ahead of eczema that keeps coming back
Results from 2 clinical trials of adults with moderate-to-severe eczema at 16 weeks:
almost clear skin
37% on DUPIXENT vs
9% not on DUPIXENT
itch reduction
38% on DUPIXENT vs
11% not on DUPIXENT
Almost half of adults saw
skin
48% on DUPIXENT vs 13% not on DUPIXENT
Many saw
skin
33% on DUPIXENT vs 7% not on DUPIXENT
Have you spoken to a dermatologist or allergist about DUPIXENT?
Great! Sign up for DUPIXENT MyWay®, our patient support program, to access program services and get one-on-one support every step of the way, including help with filling your first prescription.
If you're still struggling with moderate-to-severe eczema, maybe it's time to change the conversation.
Answer a few short questions about your eczema symptoms and treatment goals. We’ll turn it into a personalized discussion guide you can use at your next visit.
Specialists like dermatologists or allergists often have more experience treating moderate-to-severe eczema and can help create a personalized care plan for you.
Long-term results with DUPIXENT
Results from a clinical trial that studied DUPIXENT for 12 months:
Long-lasting clearer skin
At 4 months: 39% on DUPIXENT +
TCS vs 12% on TCS only
At both 4 months and 12 months:
22% on DUPIXENT + TCS vs 7%
on TCS only
Itch relief that lasts
At 12 months: 51% on DUPIXENT + TCS
vs 13% on TCS only
Fast itch relief after the first dose
At 2 weeks: 18% on DUPIXENT +
TCS vs 8% on TCS only
TCS, topical corticosteroids.
DEMONSTRATED
long-term safety profile
Long-term safety results for adults continuing on DUPIXENT in an extension study through 5 years were consistent with those seen in the original clinical trials.
Most common side effects include:
- Injection site reactions
- Eye problems, including eye and eyelid inflammation, redness, swelling, itching, eye infection, dry eye, and blurred vision
- Cold sores in your mouth or on your lips
- High count of a certain white blood cell (eosinophilia)
View the possible side effects of DUPIXENT
What you should know about the adult patients in these studies:
atopic dermatitis
atopic dermatitis
of the disease
surface
area
involvement
Stay connected
Feel like you're struggling with your symptoms?
Sign up for information that can help.