View BEFORE AND AFTER Photos IN YOUNG CHILDREN
to see results

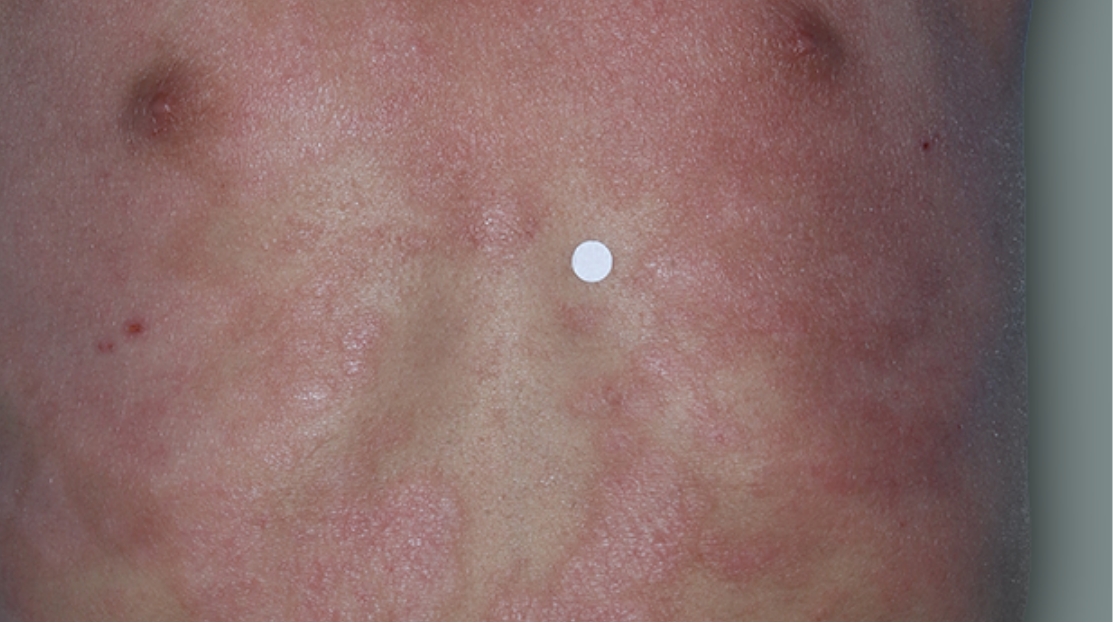
Before
Treatment
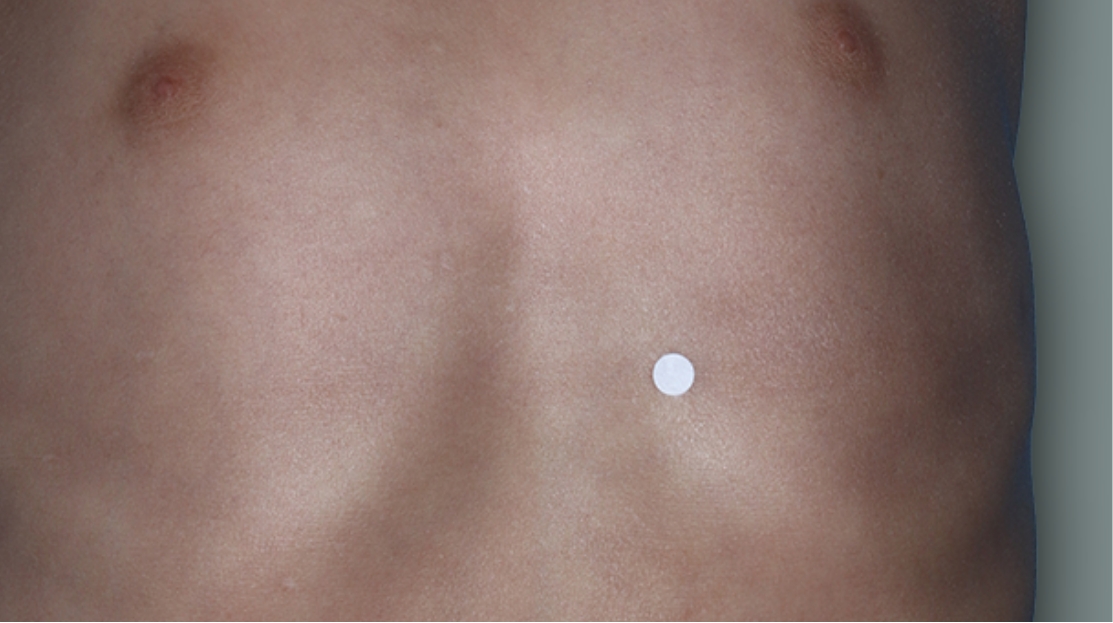
AFTER TREATMENT
With
DUPIXENT at
16 Weeks
Actual 4-year-old clinical trial patient treated with DUPIXENT and a topical corticosteroid. This patient met clinical trial criteria for clear or almost clear skin. Individual results may vary.
Proven relief from eczema symptoms
Results from a clinical trial of patients ages 6 months to 5 years with moderate-to-severe eczema at 16 weeks:
ALMOST CLEAR SKIN
28% on DUPIXENT + TCS
vs 4% on TCS only
48% on DUPIXENT + TCS
vs 9% on TCS only
of young children
53% on DUPIXENT + TCS vs 11% on TCS only
TCS, topical corticosteroids.
LONG-TERM
SAFETY RESULTS
IN YOUNG CHILDREN
Long-term safety results in young children continuing on DUPIXENT with or without TCS in the pediatric extension study through 1 year were consistent with the adult clinical trials. In addition, hand-foot-and-mouth disease and warts were reported. In these reported cases, patients continued treatment with DUPIXENT.
Most common side effects include:
- Injection site reactions
- Eye problems, include eye and eyelid inflammation, redness, swelling, itching, eye infection, dry eye, and blurred vision
- Cold sores in your mouth or on your lips
- High count of a certain white blood cell (eosinophilia)
View the possible side effects of DUPIXENT
What you should know about the patients in this study:
atopic dermatitis
atopic dermatitis
surface
area
involvement
Have you spoken to a dermatologist or allergist about DUPIXENT?
Great! Sign up for DUPIXENT MyWay®, our patient support program, to access program benefits and get one-on-one support every step of the way, including help with filling your first prescription.
If you’re still struggling with moderate-to-severe eczema, maybe it’s time to change the conversation.
Answer a few short questions about your eczema symptoms and treatment goals. We’ll turn it into a personalized guide you can use at your next visit.
Specialists like dermatologists or allergists often have more experience treating moderate-to-severe eczema and can help create a personalized care plan for you.
Stay connected
Feel like you're struggling with your symptoms?
Sign up for information that can help.