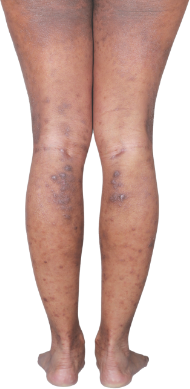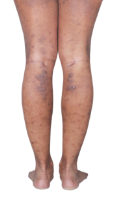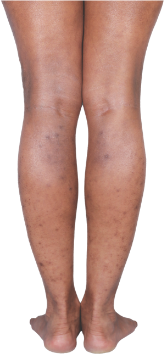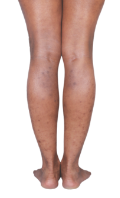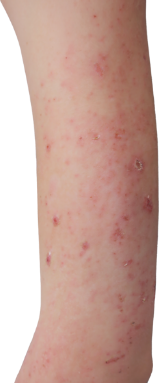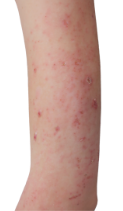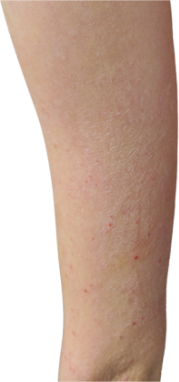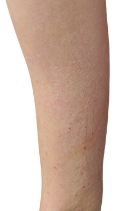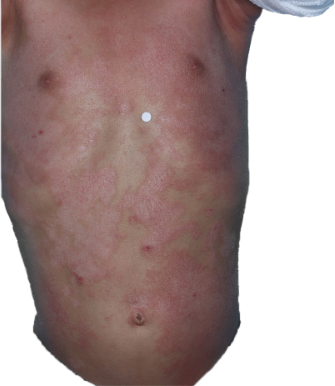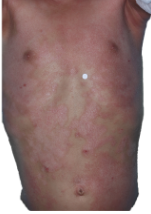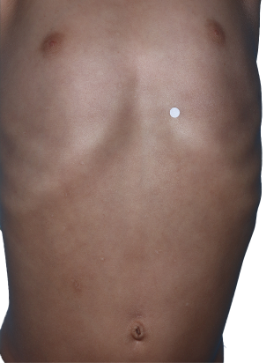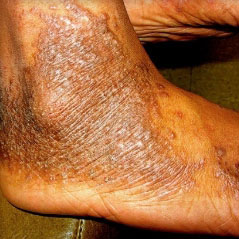
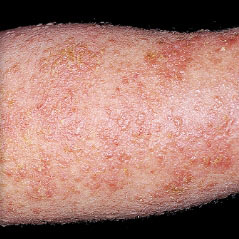
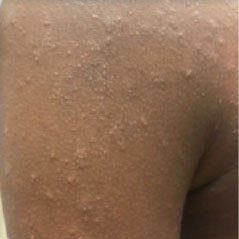
HOW DOES ECZEMA LOOK ON YOUR SKIN?

View photos from real eczema patients to better understand what moderate-to-severe eczema may look like across various skin tones.
Stay connected
Feel like you’re struggling with your symptoms?
Sign up for information that can help.

As Little As $0 Copay
The DUPIXENT MyWay® Copay Card may help eligible, commercially insured patients cover the out-of-pocket cost of DUPIXENT and pay as little as $0* in copay per fill of DUPIXENT. If you’re eligible, you can sign up and your card will be sent via email. Terms & Restrictions apply.
View full copay card Terms & Restrictions.
*Subject to the program maximum per patient per calendar year. Approval is not guaranteed. THIS IS NOT INSURANCE. Not valid for prescriptions paid, in whole or in part, by Medicaid, Medicare, VA, DOD, TRICARE, or other federal or state programs, including any state pharmaceutical assistance programs. This program is not valid where prohibited by law, taxed, or restricted. DUPIXENT MyWay reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Any savings provided by the program may vary depending on patients’ out-of-pocket costs. The program is intended to help patients afford DUPIXENT. Patients may have insurance plans that attempt to dilute the impact of the assistance available under the program. In those situations, the program may change its terms. Additional terms and conditions apply.
WANT TO LEARN MORE?