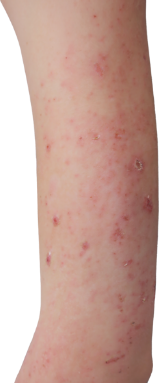
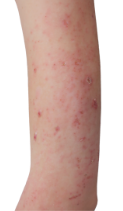
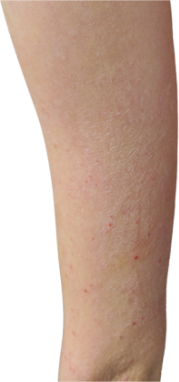
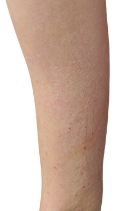
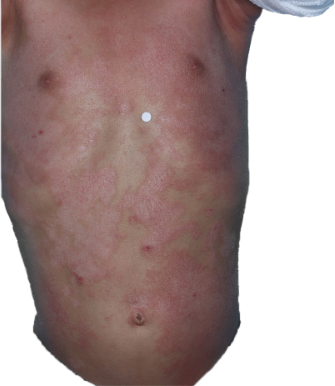
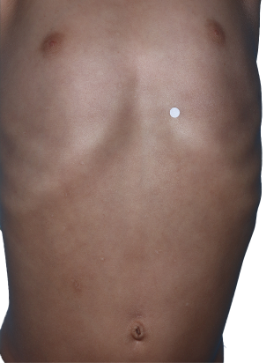
Targets a source of eczema, not just the symptoms
DUPIXENT works under the skin, continuously treating eczema—even between flare-ups.
A healthy immune
system produces little
to no inflammation on
the skin.
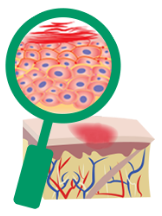
Itching, rashes, and
eczema flare-ups can
happen because your
immune system is
overactive and
overreacting to even
small triggers.
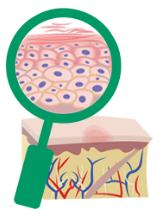
treated with DUPIXENT
DUPIXENT proactively targets a source of underlying inflammation in your body that causes itching and rashes on the surface of your skin.
What TO KNOW ABOUT DUPIXENT?
- A biologic that CONTINUOUSLY TREATS ECZEMA FROM
THE INSIDE, even between flare-ups - ONLY BIOLOGIC approved to treat moderate-to-severe
eczema in patients as young as 6 months old - Treats both MODERATE AND SEVERE ECZEMA when topical
prescription therapies aren’t enough
- NOT A STEROID or an immunosuppressant
- DOES NOT REQUIRE initial or routine blood work (according to the Prescribing Information)
- Taken only ONCE OR TWICE A MONTH (based on age and weight)*
*Adults take DUPIXENT every 2 weeks. Certain children may be eligible to take DUPIXENT once every 4 weeks. See Dosing Page for more information.
See How DUPIXENT Works
What sets DUPIXENT apart? Discover how it helps block a key source of inflammation that can cause eczema.
WANT TO LEARN MORE?
Find easy access to important information about DUPIXENT